Superficial Siderosis of the Central Nervous System Originating from the Thoracic Spine: A Case Report
Article information
Abstract
Superficial siderosis of the central nervous system(SSCNS) is a rare disease characterized by hemosiderin deposition on the surface of the central nervous system. We report a case of SSCNS originating from the thoracic spine, presenting with neurological deficits including, sensorineuronal hearing loss, ataxia, and corticospinal and dorsal column tract signs. The patient underwent dural repair with an artificial dural patch. Clinical findings were elicited by neurological examination, imaging studies, and intraoperative findings, and these were addressed through literature review.
INTRODUCTION
Superficial siderosis of the central nervous system(SSCNS) is an uncommon and unrecognized disorder caused by hemosiderin deposition in the subarachnoid space. This may lead to progressive neurological deficits, such as sensorineuronal hearing loss, cerebellar ataxia, signs of corticospinal tract dysfunction7151617).
Since the first pathologic clinical report of SSCNS was a case presented by Hamill in 1908, there have been approximately 300 reported cases of SSCNS in the literature3). To date, only 21 cases of SSCNS originating from the spinal cord have been reported2619).
Hemosiderin deposition in the subarachnoid space results from various pathologies, including tumors, meningocele and pseudomeningocele, vascular malformations, trauma, subarachnoid hemorrhage, spinal surgery, and brachial plexus, or nerve root injury. Spontaneous intracranial hypotension has also been associated with superficial siderosis20).
Here, we report a case of SSCNS originating from the thoracic spine with a dural defect. The patient presented with progressive gait disturbance after falling off a ladder. We also discuss the clinical symptoms, laboratory tests, imaging findings, and the association of SSCNS and dural defects.
CASE REPORT
A 55-year-old male developed progressive gait disturbance after falling off a ladder in March 2011. He felt "off balance" and noticed over the next two years an increasing difficulty walking. He had also developed difficulty voiding and tinnitus in both ears. He had visited several hospitals and undergone studies with computed tomographic angiography, transfemoral cerebral angiography, and magnetic resonance imaging (MRI) examinations. At that time, abnormal findings were not detected.
In April 2014, he was referred to Samsung Medical Center for ongoing neurological abnormalities. The patient's neurological exam was notable for dysmetria on heel-to-shin testing, which was markedly worse with the eyes closed. Tandem gait was noted to be unsteady, with a positive Romberg. Pinprick and temperature sensation were reduced in the distal lower extremities. Strength was 5/5 bilaterally in both lower extremities. Urodynamic studies revealed severe underactivity of the detrusor. Examination of the cerebrospinal fluid found the red blood cell count elevated at 15,250/µL along with a single siderophage.
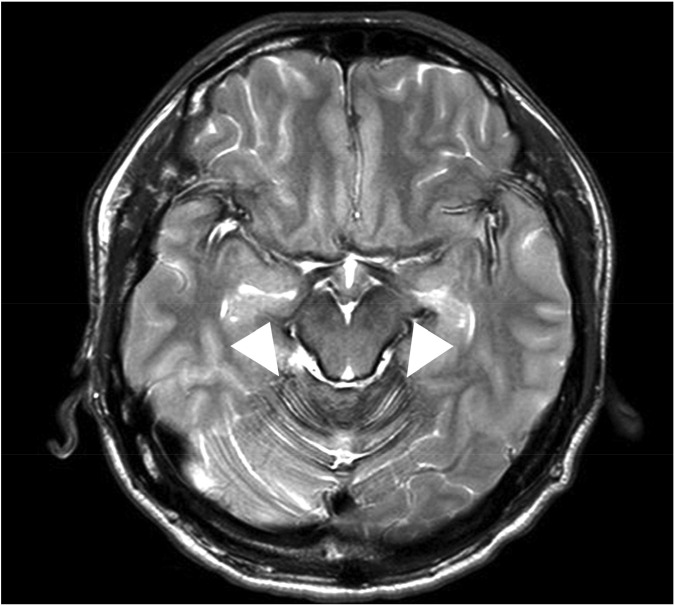
The patient underwent lumbar spine MRI at Samsung Medical Center, when the T2-weighted image (T2WI) demonstrated characteristic hypointensity outlining the spinal cord (Fig. 1). Additionally, adhesive arachnoiditis was found at the cauda equina, likely a result of previous subarachnoid hemorrhage. On brain MRI, hemosiderin deposition was seen in the sylvian fissures, posterior fossa and basal cistern (Fig. 2). CT myleography was performed to demonstrate the site of dural defect. After the skin area has been numbed, the dye is injected into the spinal sac, then the table is slowly rotated in a circular motion, first down at the head end for approximately 4 to 6 minutes, then rotated up at the head end for the same duration. The study showed a dural defect at T5, through which transdural accumulation of contrast media at the T3-T5 level was observed (Fig. 3).
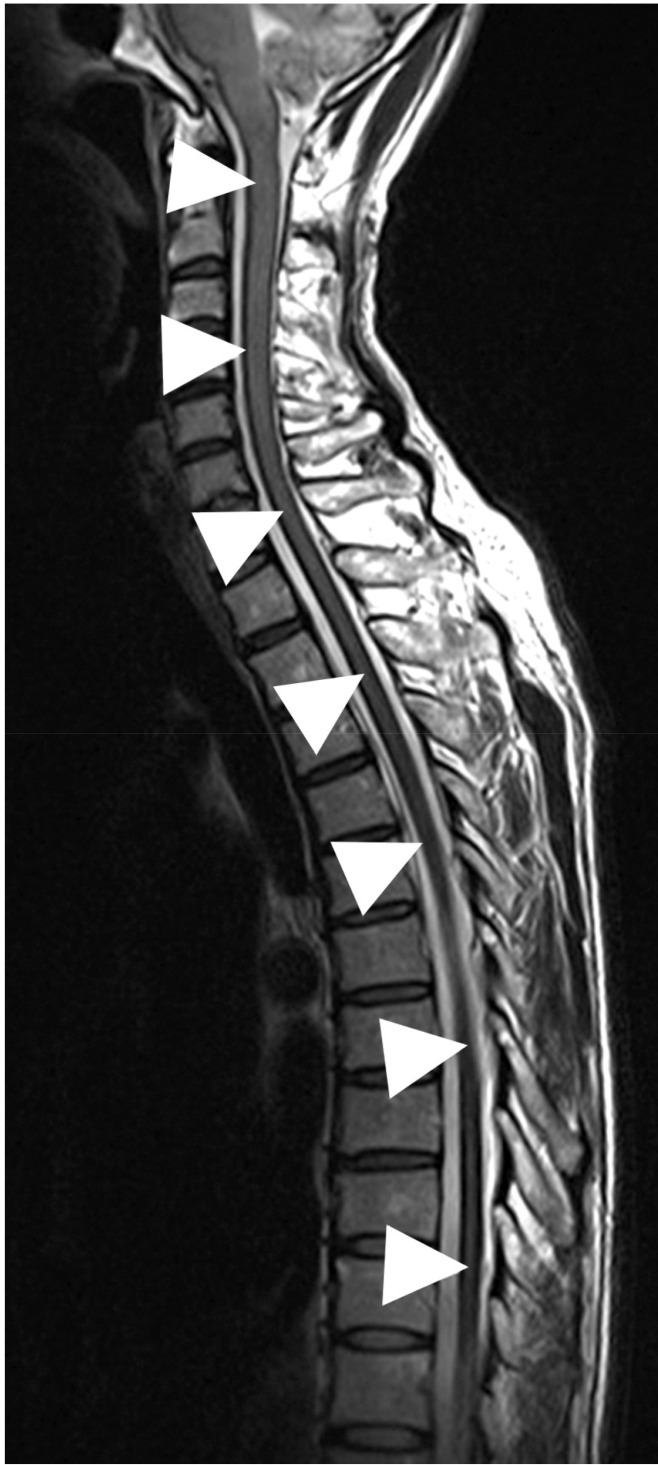
Magnetic resonance scan of the cervical spine. Marginal hypointensity along the spinal cord was seen in the T2-weighted image (arrowheads).

Magnetic resonance scan of brain. Findings of hemosiderin deposition were seen around the crest of the cerebellar folia (arrowhead).
Computed tomography (CT) myelogram of the thoracic level. Preoperative axial CT myelogram showing fluid collection in the spinal canal (arrowheads).
Under monitoring of somatosensory and motor evoked potential (MEP), the patient underwent surgery to repair the dural defect. Hemilaminectomy was performed at the left partial inferior T4 and partial superior T5. The spinal cord was carefully retracted using a Penfield No. 4 dissector (Symmetry Surgical, Antioch, TN, USA). A dural hole was visualized at the ventral portion of T5 (Fig. 4A) and fragile vessels were found surrounding the surface of the dural defect. We resected a tiny piece of arachnoid at the dural defect for histological examination, after which primary closure of the dura was attempted with 6-0 polypropylene (Ehticon, Somerville, NJ, USA), using a single stitch. Additionally, a 0.5×0.5-mm patch of artificial dura (Aesculap Inc., Central Valley, PA, USA) was anchored to the remaining dural defect (Fig. 4B). At this time, we noticed the loss of the MEP at the left lower extremity and paused the procedure for 20 minutes, before resuming closure of the dura using 6-0 polypropylene (Ehticon). We did not recover the MEP during the remainder of the procedure. Immediately after recovery from anesthesia, strength in the left lower-extremity was 0/5. By postoperative day one, the strength had improved to 1/5. After that, the patient showed graual improvement of the weakness. One month after the surgery, strength was 4+/4+ bilaterally in both lower extremities.
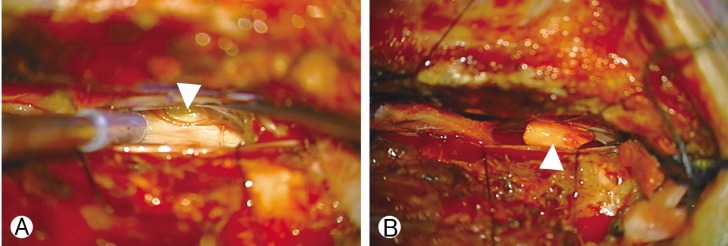
Operative microscopic view. (A) The dural hole at the ventral portion of the spinal cord (arrowhead), (B) A dural patch was anchored to the remaining dural defect (arrowhead).
Histological examination demonstrated chronic inflammation of the arachnoid membrane. Follow-up MRI at 2 months after surgery showed no significant interval change in superficial siderosis along the spinal cord. Six months after surgery, gait disturbance and voiding difficulty had progressed, though tinnitus and diplopia were slightly improved.
DISCUSSION
SSCNS is a rare condition characterized by the deposition of hemosiderin in the subpial layers of the central nervous system(CNS), as a result of chronic subarachnoid bleeding. The first pathologic clinical presentation of SSCNS was a case reported by Hamill in 1908, but only 40 cases appeared in the literature before the widespread use of MRI examination. After that, an additional 230 cases have been reported, all showing a strikingly similar T2WI hypointensity on MRI examination3). To date, only 21 cases of SSCNS originating from the spinal cord have been reported. Thirteen of these involved a spinal dural defect, while the other eight involved meningoceles26). Males are affected more frequently than females with a ratio of 2:115). One case series report, which included a review of the literature, reported that the mean age of patients with SSCNS was 59 years10).
Idiopathic bleeding is the main cause of subarachnoid bleeding, accounting for 35% of all cases; this is followed by CNS tumors (15%), head trauma (13%), and arteriovenous malformation (9%)7). Spontaneous intracranial hypotension has also been associated with superficial siderosis20).
The pathophysiology of SSCNS primarily results from hemosiderin desposition in the subaracnoid space. Hemosiderin is found within cells and appears to be a complex of ferritin, denatured ferritin and other materials. Ferritin is known to be involved in the CNS tissue response to CSF hemoglobin and hemoglobin breakdown products13). Animal studies suggest that the ferritin repressor protein known as immunoreactive Bergmann glia and ferritin-containing microglia are involved in the conversion of heme to ferritin to hemosiderin. Recurrent subarachnoid hemorrhage leads to an overproduction of hemoglobin degradation products, including free iron8). The free iron from hemosiderin catalyzes the oxidation of hydrogen peroxide to superoxide, causing lipid peroxidation, membrane dysfunction, and ultimately cell death. Microscopically, the end result is neuronal loss, reactive gliosis, and axonal demyelination9). Because of the microanatomy of the CNS and the specific cerebrospinal fluid flow pattern, hemosiderin deposition generally occurs in the superior vermis, crest of the cerebellar folia, basal frontal lobe, temporal cortex, brainstem, spinal cord, nerve roots, and cranial nerves I and VIII7915). In the present case, hemosiderin deposition was limited to the spinal cord and infratentorial area. Fragile vessels were found surrounding the surface of the dural defect, and were assumed to be the cause of chronic repetitive bleeding that eventually resulted in SSCNS. Even in the first series of SSCNS cases described by Bracchi et al.3), many posttraumatic events were noted. The fragile vessels developing on the unrepaired dura were implicated as the probable source of chronic microhemorrhages3).
The neurologic deficits caused by hemosiderin deposition are irreversible. The latent period of SSCNS ranges from 4 months to 30 years7). Common clinical findings include sensorineuronal hearing loss (95%), progressive cerebellar ataxia (88%), and pyramidal tract signs (76%)151617). In the present case, the patient exhibited ataxia, diplopia, voiding difficulty and bilateral tinnitus with preserved hearing. Though the current patient did not experience hearing deficits, long-term follow-up is required, as the development of sensorineuronal deafness is common.
Prior to the widespread use of MRI, the diagnosis of SSCNS depended on postmortem examination or surgical biopsy7). Currently, MRI is the diagnostic modality of choice. T2WI MRI reveals a pathognomonic linear hypointensity due to hemosiderin deposition around the brainstem, cerebellum, spinal cord, and cauda eaquina9). Recently, an association between dural defects in the spine and SSCNS has been suggested. Sometimes, a longitudinally fluid-filled collection in the spinal canal is observed with MRI. Kumar et al.11) have reported the efficacy of CT myelography in detecting the communication between the subarachnoid space and fluid collection. In the present case, CT myleography was performed to detect the site of dural defect and showed a dural defect at T5 with accumulation of contrast media (Fig. 2). Even though myelography is generally regarded as a safe procedure, close attention is necessary in the possibility of fatal complications such as seizure and rhabdomyolysis4).
Currently, there is no effective treatment for SSCNS. Medical management in the form of corticosteroids has been reportedly successful in a unique case associated with anti-Ri antibodies1). Penicilamine and large doses of vitamin C and E have also been effective in certain patients14). Treatment with deferiprone, a lipid soluble iron chelator, has reduced hemosiderin deposition, as evidenced on MRI, in a cohort of 10 patients with SSCNS. However, prospectively designed efficacy studies are necessary to determine the clinical efficacy of deferiprone in SSCNS15).
As there is no definitive treatment for SSCNS, surgical closure of the defect remains the most reliable method, effectively halting ongoing bleeding from dural defects associated with SSCNS6). However, surgery has been effective in very few patients, with many patients experiencing aggravated neurological deficiencies following surgery. In the present case, the aggravation of gait disturbance and voiding difficulty is likely to have been caused by ongoing SSCNS. Posti et al.19) performed a PubMed search using the keywords "superficial siderosis" associated with the keywords "surgery", "surgical," "operative", and "treatment". Of the 29 surgically managed cases of SSCNS, clinical improvement was seen in only 5 cases, with another 5 cases endorsing progressive symptoms following surgery. The remaining 19 cases endorsed neurological stability. Payer et al.18) recently reported that the disease can progress secondarily without rebleeding after surgical closure. Direct suture can be difficult to perform when the defect is located in the ventral portion, as in the present case. We performed additional anchoring sutures with an artificial dura, in which cases, fibrin glue, collagen sponges, and muscle or fat grafting can also be used in repair251112).
CONCLUSION
Surgical repair of the dural defect is effective to halt cerebrospinal fluid leakage and subarachnoid hemorrhaging. If there are long histories of SSCNS, the symptoms can deteriorate even after a complete dural closure. Therefore, early diagnosis, precise detection of the dural defect location, and prompt surgical repair are important for minimizing the neurologic impairment.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.