|
 |
- Search
|
|
||
Abstract
Objective
Loss of skeletal muscle mass is known to be associated with multiple morbidities. However, there is a dearth of reports on its association with lumbar lordosis and musculoskeletal pain. The aim of this study was to delineate the cross-sectional relationship between loss of skeletal muscle mass, lumbar lordosis, and chronic low back pain (CLBP).
Methods
A total of 721 medical records were reviewed, and data from 165 older subjects (over 65 years old; 81 men and 84 women) were retrospectively analyzed. Subjects were categorized into either the CLBP group (back pain for more than 6 months; 35 men and 36 women) or the control group (46 men and 48 women). The modified skeletal muscle mass index (MSMI, appendicular skeletal muscle mass [kg]/weight [kg] × 100), assessed by bioelectrical impedance analysis, and lumbar lordotic angle (LLA) were measured and compared between the CLBP group and the control group. The correlation between MSMI and LLA was investigated.
Chronic low back pain (CLBP) has become an important social issue in the era of aging as it can lead to increased medical costs, as well as, disabilities affecting the patient’s quality of life [1]. While it is hard to pinpoint a specific cause of low back pain, approximately 97% of low back pain is believed to be related to mechanical causes [2]. This has given rise to a growing body of research focusing on recovery or rehabilitation after surgical interventions for these causes [3,4], prognostic predictions for postoperative outcomes [5], and parameters associated with CLBP [6-9]. To prevent or treat CLBP, maintaining sound mechanical integrity of low back would be crucial because stability of spine depends on passive spinal column, active spinal muscles, and neural controls [10].
More recently, there has been a surge in studies investigating the various influences of sarcopenia on cardiovascular disease, metabolic disorders, and musculoskeletal disorders [7,11,12]. Considering that muscle is vital for protecting the integrity of the musculoskeletal system, there must be meaningful associations between loss of skeletal muscle mass and painful musculoskeletal conditions. Previous studies examining the relationship between regional muscle mass and CLBP reported that the cross-sectional area of the trunk and back muscles, as assessed by computed tomography (CT) or magnetic resonance imaging (MRI), were reduced in patients with CLBP [6-8,13]. In addition, biomechanical factors underlying CLBP are influenced not only by trunk and back muscles but also by extremity muscles such as large hip muscles [14,15]. Furthermore, previous studies have shown an association between CLBP and low appendicular skeletal muscle mass (ASM), as assessed by bioelectrical impedance analysis (BIA) [7,8]. This supports the hypothesis that CLBP may share a close relationship with age-related loss of skeletal muscle mass, so called sarcopenia.
The importance of healthy muscular control on spinal column was evidenced that muscular activation or training helped to prevent CLBP by providing stability or postural correction [10]. Also, altered sagittal alignment has been described as a substantially influencing factor for CLBP including symptomatic adjacent segment degeneration [5], and a meaningful relationship has been reported between CLBP and decreased lumbar lordotic angles (LLAs) [8,16-18]. Considering muscle integrity is important for maintaining normal LLA [19,20] and adequate sagittal balance [21], there may be a significant association between loss of skeletal muscle mass and CLBP, probably through altered sagittal balance such as changes of LLA.
To examine the hypothesis that there might be close relationships between skeletal muscle mass, LLA, and CLBP, this study retrospectively analyzed health-screening data to investigate any differences in muscle mass and LLA between elderly subjects with and without CLBP, and to evaluate the relationship between skeletal muscle mass and LLA.
We performed a retrospective cross-sectional study based on health-screening data of subjects aged 65 and over who had routine medical check-up at the Gangnam Health-Care Center, Seoul National University Hospital between January 1, 2012 and June 30, 2014. The routine medical check-up program is a preventive health-screening program for normal population, comprising a basic anthropometric examination, serologic tests, tumor marker studies, head and neck examination, simple radiologic studies, abdominal ultrasonography, cardiologic workups, gastrointestinal endoscopic studies, and counseling with a physician. Additionally, all medical check-up recipients underwent BIA, standing lumbar lateral radiography, and an assessment of back pain history by a physician. All of the subjects were Koreans by ethnicity and citizenship.
Subjects who had been experiencing low back pain for more than 6 months were included in the CLBP group, while subjects who reported no back pain during the past 6 months were included as study controls. Medical data were excluded from the analysis if a subject had acute low back pain newly occurred within the past 6 months, spine cancer, spinal trauma, compression fracture, rheumatoid arthritis, history of spinal surgery, or if there was no written medical record about back pain history on review of system.
This study was approved by the institutional review board of Seoul National University Hospital (IRB No. H-1404-031-568).
A lumbar spine lateral radiograph was taken, with focus on the lumbar region and at a distance of approximately 1 meter away from the subject. The subject was instructed to stand naturally while gripping a handle in front of them as lightly as possible to maintain balance. For measurements of LLA, 2 lines were drawn parallel to the upper endplate of the L1 vertebra and the lower endplate of the L5 vertebra. Perpendicular lines to each of the aforementioned lines were drawn so that their intersection formed the LLA (Fig. 1).
BIA with 8 tactile electrodes (MF-BIA8; InBody 720, Biospace, Seoul, Korea) was used to measure the impedances of 5 body segments (right arm, left arm, trunk, right leg, and left leg) separately, and using 6 different frequencies (1 kHz, 5 kHz, 50 kHz, 250 kHz, 500 kHz, 1,000 kHz). In the routine medical check-up, a trained nurse took the BIA measurements after at least 3 hours of fasting and voiding by the subject. For the measurements, subjects were instructed to be barefoot and stand upright with their feet placed on electrodes of the machine platform, and with their hands gripping on to the electrodes of the arm handles. The ASM was obtained by summing the 4 measurement values of both arms and legs, which were separately presented after calculation of muscle mass in each limb and the trunk. To compare muscle masses among subjects with different body habitus, ASM was normalized considering the body weight to generate the modified skeletal muscle mass index (MSMI), calculated as (ASM [kg]/weight [kg]× 100%).
Age, weight, height, body mass index (BMI), MSMI, and LLA were compared between the CLBP and control groups by using an independent T-test. Correlation analysis was also performed between LLA and MSMI using Pearson correlation coefficients. All statistical analyses were performed separately for male and female subjects as normal ranges of MSMI and LLA are reported to differ significantly based on sex. In addition, a subgroup analysis was performed in which the total subject was divided into 2 age groups: those under 70 and those aged 70 or over.
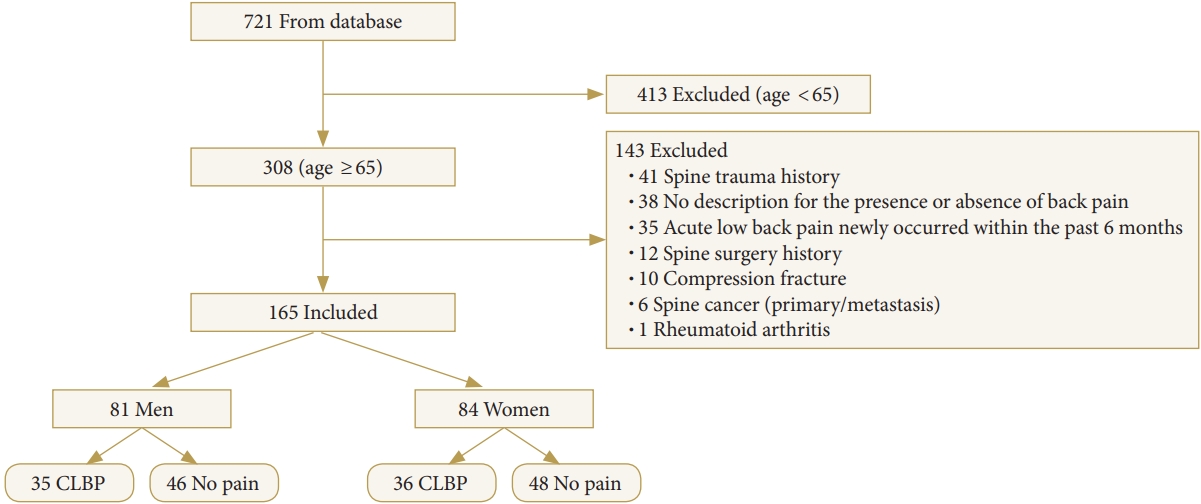
Data of 308 subjects over the age of 65 were extracted from a dataset of 721 subjects who underwent BIA and lumbar spine radiography on the same day. Subjects who did not meet the study-specific inclusion criteria were excluded (n=143). Subjects with a history of spine trauma (n=41), no description for the presence or absence of back pain (n=38), back pain occurrence within 6 months (n=35), history of spine surgery (n=12), compression fractures (n=10), spine cancer (n=6), and rheumatoid arthritis (n=1) were excluded from the analysis. Data of the remaining 165 subjects were included in the final analysis. The final analysis included data of 81 men and 84 women categorized into either the CLBP or the control group (Fig. 2).
Women with CLBP were significantly shorter than those in the control group (p=0.02); however, there were no significant differences in age, weight, or BMI between the 2 study groups for men or women (Table 1).
Fig. 3A illustrates the differences in LLA between CLBP and control groups. A significantly lower LLA was observed in men from the CLBP group (29.8°±10.6°) compared to those in the control group (37.1°±8.5°, p=0.001). Similarly, LLA was smaller in women with CLBP in comparison to their counterpart controls (32.1°±11.2° vs. 38.3°±9.2°, p=0.006). In the subgroup analysis, participants were divided into 2 groups: those under 70 years of age and those aged 70 or over. For men under the age of 70, the CLBP group had a statistically significant lower LLA than the control group (29.4°±10.2° vs. 38.8°±8.7°, p=0.002) (Fig. 3B). For women aged 70 or over, the CLBP group had a statistically significant lower LLA than the control group (30.3°±11.2° vs. 37.7°±8.8°, p=0.046) (Fig. 3C) (see Supplementary Tables 1 and 2 for details).
Fig. 3D illustrates the difference in MSMI between CLBP and control groups. Male CLBP subjects had lower MSMI (31.2%±1.7%) than male control subjects (32.3%±1.9%, p=0.008). Female subjects also showed similar results considering MSMI values (26.1%±1.9% vs. 27.1%±2.1%, p=0.021) for the CLBP and control groups, respectively. In the subgroup analysis based on age into 2 groups (< 70 and ≥ 70), the MSMI value was significantly lower in the CLBP group compared to the control group for women under 70 (25.8%±1.9% vs. 27.5%±2.3%; p=0.007) (Fig. 3E) (see Supplementary Tables 1 and 2 for details).
Positive correlations were observed between the LLA and the MSMI both in the male (r=0.220, p=0.048) and female (r=0.225, p=0.040) subjects (Fig. 4A, D). In the age-stratified subgroup analysis, a significant positive correlation between LLA and MSMI was observed in women under the age of 70 (r=0.336, p=0.015) (Fig. 4E).
In the present study, retrospective analysis of routine medical check-up data of subjects older than 65 years revealed decreased LLA and MSMI in CLBP subjects. The LLA and MSMI of the CLBP group was significantly lower than that of the control group. A significant positive relationship between LLA and MSMI were also observed.
The results of this study, which showed significantly lower MSMI in the CLBP group, were in line with previous studies that have demonstrated the relationship between CLBP and the quality and quantity of truncal and appendicular muscle. Danneels et al. reported that the cross-sectional areas of the psoas and multifidus muscles below the L4 endplate were decreased in patients with CLBP [13]. Hicks et al. [6] and Baek et al. [9] also reported that increased fat infiltration of the trunk muscles was significantly associated with CLBP, although the area per se of the trunk muscles did not show a significant difference. Additionally, Eguchi et al. [11] demonstrated that the severity of clinical symptoms, as assessed by the Roland-Morris Disability Questionnaire, was significantly correlated with ASM. Considering previous findings on the association between truncal muscle and ASM [12], which is used as a criterion for sarcopenia, the present results showing that the CLBP group had lower MSMI could be interpreted in 2 different ways.
Firstly, it may reflect the fact that the mechanical integrity of the lumbar spine is influenced, not only by the paraspinal and abdominal muscles, but also by whole body muscles including the proximal limb muscles. Leinonen et al. [15] reported that activity of the gluteal muscles was reduced in patients with CLBP during flexion-extension cycle. Increase of fatigability in the gluteus maximus muscle was also noted in CLBP patients through an electromyography fatigue analysis [14]. Furthermore, the evidence that multiple kinetic chains were involved in CLBP has also been supported by the study by Kim et al. [22], which showed increased activity of contralateral latissimus dorsi, ipsilateral gluteus maximus, and ipsilateral biceps femoris in patients with CLBP during hip extension test. In addition, a previous study by Eguchi et al. [11] demonstrated that ASM was associated with posterior pelvic tilt. This research also found a correlation between low back pain and both the ASM and posterior pelvic tilt. This suggests that decreased skeletal muscle mass may influence low back pain through its interactions with sagittal alignment. Based on this evidence, the lower MSMI in the CLBP group implicates that loss of muscle mass may predispose elderly people to CLBP.
Secondly, lower MSMI in the CLBP group may be consequential of chronic pain endured rather than a causative factor. It has been reported that chronic musculoskeletal conditions prevented patients from maintaining physical activities [23]. However, deconditioning effects of CLBP in our results might not have been substantial because the data were collected from a preventive health-screening program for normal population, indicating that the pain severity of CLBP in this study might not be severe enough to make the subjects seek medical service. It limits the interpretation of the results that the pain severity was not assessed in this study. While it is not possible to delineate causal relationships in this cross-sectional analysis, a relationship between CLBP and decreased muscle mass is intriguing.
In the diagnosis of sarcopenia, skeletal muscle mass can be quantified by measuring ASM using dual-energy x-ray absorptiometry (DXA) or BIA [24,25]. To appreciate variations in the body habitus of each person, European Working Group on Sarcopenia in Older People (EWGSOP) and Asian Working Group for Sarcopenia (AWGS) have presented cutoff values for the MSMI based on a height-adjusted (ASM [kg]/height [m]2) or weightadjusted (ASM [kg]/weight [kg] × 100) method [24,25]. Between the 2 methods, the weight-adjusted method is preferred in elderly people of Asian origin, as it has previously been reported that the height-adjusted method had a tendency to underestimate the prevalence of sarcopenic obesity and sarcopenia, and did not correspond well with measurements of physical function in elderly Asians [26]. In this study, we measured MSMI using the weight-adjusted method.
As the medical check-up program did not include measurements of muscle strength or performance, we could not apply the criteria of sarcopenia advocated by EWGSOP or AWGS. Nevertheless, when we applied the cutoff values of MSMI for sarcopenia from a previous study to compare with the Korean population [27], it is intriguing that the CLBP group in the present study had a substantially higher prevalence of sarcopenia (17.1% for men and 13.9% for women) than the control group (2.2% for men and 0% for women). The prevalence of sarcopenia in subjects with CLBP in this study tended to be higher than normal age-matched population, in which it was 9.7% and 11.8%, respectively [27]. This suggests a meaningful cross-sectional association between sarcopenia and CLBP.
It is of note that the prevalence of sarcopenia in our subjects was substantially low compared with those of other cohorts including the Fourth Korean National Health and Nutrition Examination Surveys (KNHANES IV) conducted in 2008–2009, which reported approximately 9.7% for man and 11.8% for women [27]. The authors speculate that this finding may be related with the fact that the subjects included in our study had rather higher socio-economic status to have lower risks of sarcopenia [28] because all of them were willing and able to pay more than $1,500 for their annual health checkups either by themselves or through employing companies.
Despite numerous studies correlating sagittal alignment with CLBP and associated quality of life [8,17,29], the relationship between low back pain and LLA is yet to be clarified as there are still conflicting reports among previous studies [16,17,30-32]. Christie et al. [30] reported that LLA was increased in patients with CLBP and thus it would be one of the causes of back pain. On the other hand, Tsuji et al. [32] reported that LLA was decreased by approximately 4 degrees in patients with CLBP regardless of sex or age. Jackson and McManus [17] also argued that the LLA was decreased in patients with CLBP, especially in the distal segment lordosis. There have been several studies that also reported no significant tendency, either decreasing or increasing, in LLA depending on CLBP [33], acute low back pain (LBP) and chronic LBP [31]. Hansson et al. [31] reported that there was no change in LLA in acute LBP, as well as, chronic LBP, and Pope et al. [34] reported that there was no difference in LLA between the group that did not experience LBP, and groups with moderate back pain or severe back pain.
Table 2 provides a summary of previous published reports on the relationship between LLA and LBP, and an interesting association is noted between the age of participants and changes in LLA; in older subjects, there was a stronger tendency to observe decreases in LLA in LBP groups. This association is consistent with our study, which analyzed data from subjects older than 65 years, and found significantly decreased LLA in our CLBP group than the controls. Because the control groups were taller than the CLBP groups especially in female subjects, the possibility of confounding effects from height difference on LLA was tested by calculating correlations between the heights and LLA in our data to find little associations in the male (r=0.087, p=0.440) and female (r=0.044, p=0.691). This finding suggests that alterations of sagittal balance in the lumbar spine may operate differently in LBP of younger versus older population. In younger people who have minimal or no disc degeneration, decreases in LLA would hardly occur in LBP conditions. Instead, LBP may trigger paraspinal muscle spasm [35] which could result in an increased LLA. However, because advanced disc degeneration is one of the predominant causes of CLBP in the elderly, severe degeneration would decrease LLA and predispose to CLBP [36,37]. This speculation draws a hypothesis that preserving LLA may have preventive or therapeutic effects on CLBP in an elderly population. Nevertheless, further longitudinal studies incorporating additional radiologic parameters reflecting sagittal alignment, such as pelvic tilt and sagittal vertical axis, to substantiate this hypothesis are required.
It is noteworthy that the present study revealed significant correlations between LLA and MSMI both in male and female subjects. Proper function and integrity of trunk muscles are known to be associated with maintaining LLA [19,20] and compensating for sagittal balance [21]. In conjunction with previous findings showing a relation between ASM and posterior pelvic tilt [11], the substantial association observed in this study between LLA and MSMI suggests that a loss of muscle mass may lead to a decrease in LLA in the elderly population. However, in this study, the association between LLA and MSMI was observed less in subjects aged 70 years and older compared to those under 70. These findings might be related to a possible presence of coexisting spinal sarcopenia in the elderly. Previous research by Kim et al. [38] reported an association between spinal sarcopenia and spinal sagittal balance in community-dwelling elderly women (mean age, 76.8 years). Also, the study of Eguchi et al. [11] on community-dwelling elderly women (mean age, 74 years) demonstrated that ASM and truncal muscle mass were significantly associated with pelvic tilt. A common thread across these studies is that the sagittal alignment of the subjects was significantly associated with the truncal skeletal muscle index. Considering the constraints of our retrospective approach, which did not include an evaluation of the paraspinal muscle, careful consideration should be given to this factor when interpreting the results.
Nevertheless, the cross-sectional design of this study inherently presents challenges in establishing causal relationships; however, the existence of potential interrelationships between LLA, muscle mass, and CLBP is suggested. There would be a series of vicious cycle, for instance, in elderly patients, reduction in skeletal muscle mass associated with decreased LLA could lead to CLBP, while CLBP could in turn compromise mobility and further exacerbate loss of muscle mass to jeopardize remaining LLA. Further delineating the interrelationships between loss of muscle mass, LLA, and CLBP could therefore have important implications for the treatment or prevention of morbidity in the elderly.
This study had several limitations. First, since the present study is cross-sectional, it is not clear enough to characterize causal relationships among each parameter. Second, due to the constraints of a retrospective study, we were unable to evaluate the cross-sectional area of truncal muscles through CT/MRI images. Many previous studies have analyzed the relationship between truncal muscle mass and LBP using axial CT/MRI images [6,9]. However, there is insufficient evidence to confirm that ASM, as evaluated by bioimpedance, consistently corresponds to truncal muscle mass. Therefore, the findings of this study should also be contemplated in conjunction with previous findings on the association between proximal limb muscles and the mechanical integrity of the lumbar spine [11,14,15,22]. In addition, the severity, location, and chronicity of CLBP and other associated medical conditions such as osteoporosis, other radiological parameters were not assessed and quantified to better focus on our research question. Series of future studies analyzing relationships of truncal muscle mass, osteoporosis, and other radiologic parameters with CLBP and LLA will help to understand the multifactorial natures of sarcopenia and CLBP. The third limitation would be using BIA instead of DXA which is known to be more accurate in measuring fat and fat free body mass [39]. However, through recent improvement of technology and stratification of data, BIA has become an accepted tool for measuring mucle mass as used in European [24] and Asian consensus on definition and diagnosis stated in the Asian Working Group for Sarcopenia [25]. Moreover, all of the participants had a standardized condition in terms of food intake and measurement time in a day because BIA measurement was done early in the morning as the first part of their medical check-up after fasting (nil-per-os) at least 8 hours by midnight null-per-oral state. Especailly, the eight-polar BIA used in this study is known to offer accurate measurements of total and appendicular body composition as DXA [40].
The current retrospective study revealed that reduced skeletal muscle mass and decreased lumbar lordosis were associated with CLBP in the elderly. Furthermore, positive correlations were observed between skeletal muscle mass and LLA, suggesting that these factors should be considered when planning therapeutic interventions. Future research involving a larger population, including patients with very severe LBP, is warranted to determine the causal relationship.
Supplementary Material
Supplementary Tables 1-2 can be found via https://doi.org/10.14245/ns.2346494.247.
Supplementary Table 1.
Characteristics of subjects under the age of 70
Supplementary Table 2.
Characteristics of subjects aged 70 or over
NOTES
Fig. 1.
Measurement of the lumbar lordotic angle. The lumbar lordotic angle (*) is formed by 2 perpendicular lines of tangents drawn along the upper endplate of L1 and the lower endplate of L5.

Fig. 3.
Differences in LLA and MSMI between CLBP and control group. (A–C) Differences in the lumbar lordotic angle and (D–F) the MSMI between the CLBP and control groups. (A, D) Total subject population, (B, E) subjects aged 65 to less than 70, and (C, F) subjects aged 70 and above. The numbers in the bars are mean values, and the whiskers denote standard deviation. LLA, lumbar lordotic angle; MSMI, modified skeletal muscle mass index; CLBP, chronic low back pain. *p < 0.05.
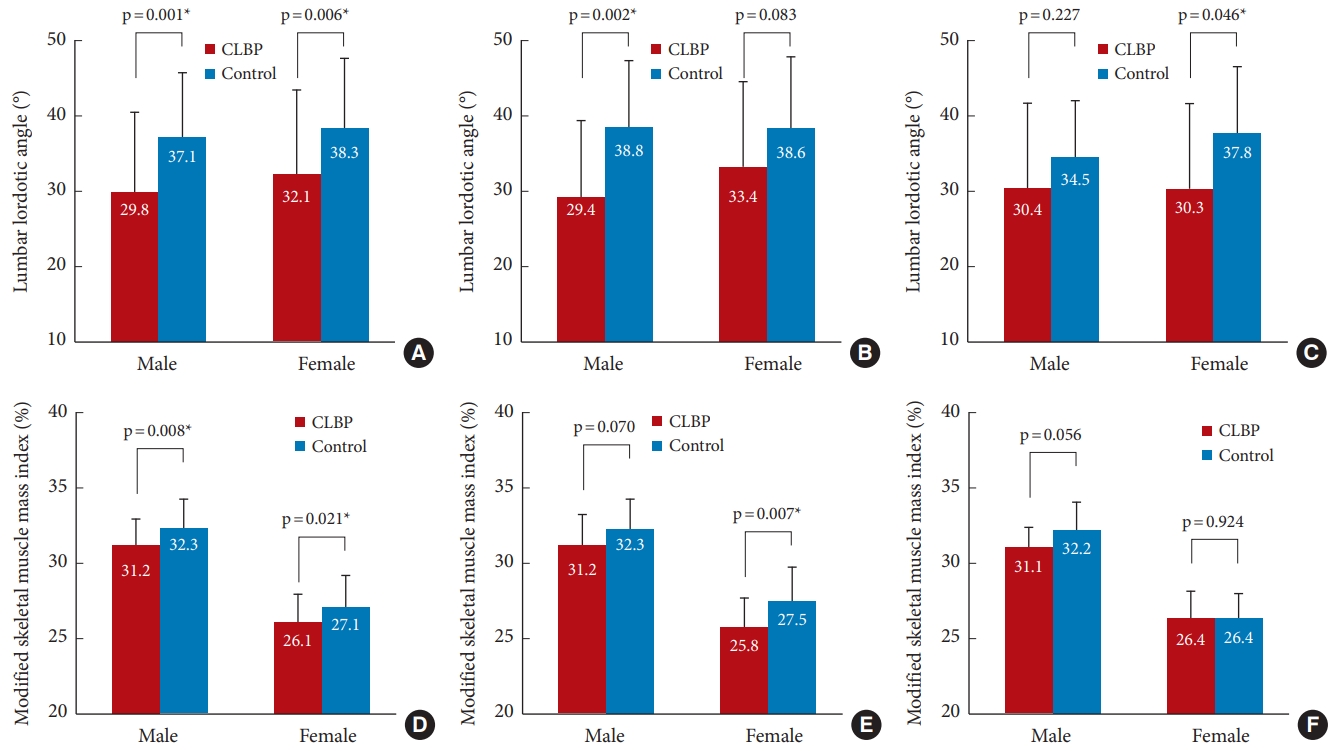
Fig. 4.
Correlation between LLA and MSMI. The Pearson correlation coefficient between the LLA and the MSMI for men (A–C) and women (D–F). (A, D) Total subject population, (B, E) subjects aged 65 to less than 70, and (C, F) subjects aged 70 and above. LLA, lumbar lordotic angle; MSMI, modified skeletal muscle mass index; CLBP, chronic low back pain. *p < 0.05.
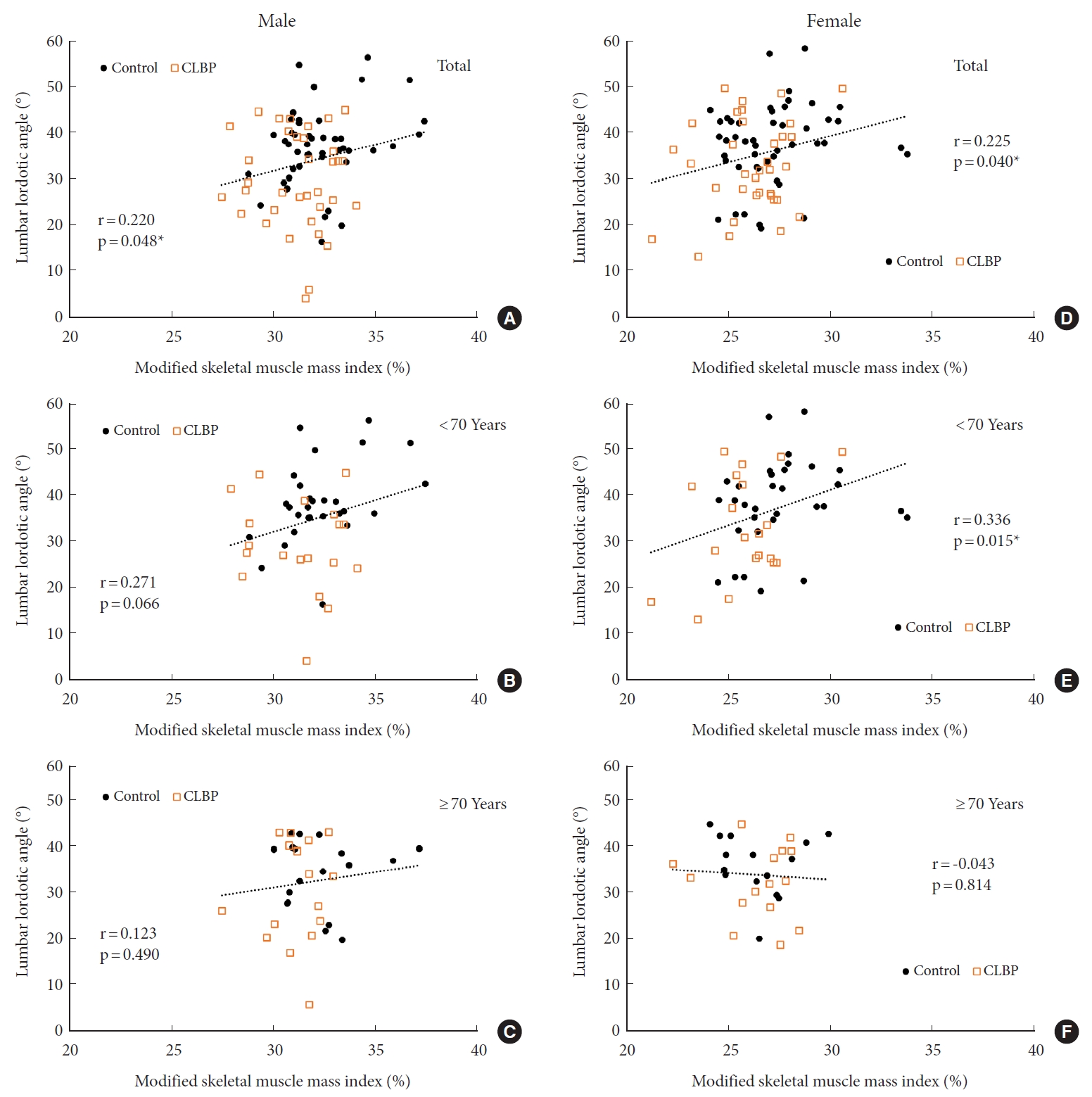
Table 1.
Subject characteristics at the time of study entry
| Characteristic |
Men (n=81) |
Women (n = 84) |
||||
|---|---|---|---|---|---|---|
| CLBP (n=35) | Control (n=46) | p-value | CLBP (n = 36) | Control (n = 48) | p-value | |
| Age (yr) | 70 ± 3.9 | 69.6 ± 5.1 | 0.657 | 69.7 ± 3.6 | 68.6 ± 2.6 | 0.135 |
| Weight (kg) | 66.9 ± 7.4 | 68.4 ± 7.5 | 0.411 | 54.7 ± 8.4 | 54.3 ± 6.6 | 0.776 |
| Height (cm) | 167.3 ± 4.7 | 168.1 ± 4.8 | 0.483 | 154.4 ± 5.1 | 156.9 ± 5.0 | 0.020* |
| BMI (kg/m2) | 23.9 ± 2.4 | 24.2 ± 2.2 | 0.617 | 22.9 ± 2.8 | 22.0 ± 2.5 | 0.130 |
Table 2.
Published reports on the relationship between lumbar lordotic angle and low back pain
| Study | Measuring modality | Subgroup | Age (yr) | Results |
|---|---|---|---|---|
| Christie et al. [30] | Photograph | LBP 39, control 20 | 18–46 | Increased LLA in LBP |
| Pope et al. [34] | X-ray | Severe LBP 71, moderate LBP 144, control 106 | 18–55 | No difference |
| Hansson et al. [31] | X-ray | CLBP 200, 1st injury 200, control 200 | 20–63 | No difference |
| Jackson and McManus [17] | X-ray | LBP 100, control 100 | 20–65 | Decreased LLA in LBP |
| Murrie et al. [33] | MRI | LBP 27, control 29 | 26–65 | No difference |
| Korovessis et al. [18] | X-ray | LBP 120, control 120 | 20–79 | Decreased LLA in LBP 60s |
| Itoi [16] | X-ray | LBP 75, control 25 | 48–89 | Decreased LLA in LBP |
| Sakai et al. [8] | X-ray | CLBP 203, control 683 | ≥ 65 | Decreased LLA in CLBP |
REFERENCES
1. Weiner DK, Kim YS, Bonino P, et al. Low back pain in older adults: are we utilizing healthcare resources wisely? Pain Med 2006;7:143-50.


3. Chang HK, Huang M, Wu JC, et al. Less opioid consumption with enhanced recovery after surgery transforaminal lumbar interbody fusion (TLIF): a comparison to standard minimally-invasive TLIF. Neurospine 2020;17:228.




4. Cheng H, Liu J, Shi L, et al. The rehabilitation-related effects on the fear, pain, and disability of patients with lumbar fusion surgery: a systematic review and meta-analysis. Neurospine 2023;20:278-89.




5. Yoon SG, Lee HC, Lee SM. Pelvic incidence–lumbar lordosis mismatch is predisposed to adjacent segment degeneration after single-level anterior lumbar interbody fusion: a retrospective case-control study. Neurospine 2023;20:301-7.




6. Hicks GE, Simonsick EM, Harris TB, et al. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2005;60:882-7.


7. Sakai Y, Matsui H, Ito S, et al. Sarcopenia in elderly patients with chronic low back pain. Osteoporos Sarcopenia 2017;3:195-200.



8. Sakai Y, Wakao N, Matsui H, et al. Clinical characteristics of geriatric patients with non-specific chronic low back pain. Sci Rep 2022;12:1286.




9. Baek S, Park HW, Kim G. Associations between trunk muscle/fat composition, narrowing lumbar disc space, and low back pain in middle-aged farmers: a cross-sectional study. Ann Rehabil Med 2022;46:122-32.




10. Ikeda DM, McGill SM. Can altering motions, postures, and loads provide immediate low back pain relief: a study of 4 cases investigating spine load, posture, and stability. Spine (Phila Pa 1976) 2012;37:E1469-75.

11. Eguchi Y, Suzuki M, Yamanaka H, et al. Associations between sarcopenia and degenerative lumbar scoliosis in older women. Scoliosis Spinal Disord 2017;12:9.




12. Seo HS, Lee H, Kim S, et al. Paravertebral muscles as indexes of sarcopenia and sarcopenic obesity: comparison with imaging and muscle function indexes and impact on cardiovascular and metabolic disorders. Am J Roentgenol 2021;216:1596-606.

13. Danneels LA, Vanderstraeten GG, Cambier DC, et al. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J 2000;9:266-72.




14. Kankaanpää M, Taimela S, Laaksonen D, et al. Back and hip extensor fatigability in chronic low back pain patients and controls. Arch Phys Med Rehabil 1998;79:412-7.


15. Leinonen V, Kankaanpää M, Airaksinen O, et al. Back and hip extensor activities during trunk flexion/extension: effects of low back pain and rehabilitation. Arch Phys Med Rehabil 2000;81:32-7.


16. Itoi E. Roentgenographic analysis of posture in spinal osteoporotics. Spine (Phila Pa 1976) 1991;16:750-6.


17. Jackson RP, McManus AC. Radiographic analysis of sagittal plane alignment and balance in standing volunteers and patients with low back pain matched for age, sex, and size. A prospective controlled clinical study. Spine (Phila Pa 1976) 1994;19:1611-8.

18. Korovessis P, Stamatakis M, Baikousis A. Segmental roentgenographic analysis of vertebral inclination on sagittal plane in asymptomatic versus chronic low back pain patients. J Spinal Disord 1999;12:131-7.


19. Kim HJ, Chung S, Kim S, et al. Influences of trunk muscles on lumbar lordosis and sacral angle. Eur Spine J 2006;15:409-14.



20. Youdas JW, Garrett TR, Egan KS, et al. Lumbar lordosis and pelvic inclination in adults with chronic low back pain. Phys Ther 2000;80:261-75.



21. Bae J, Sathe A, Lee SM, et al. Correlation of paraspinal muscle mass with decompensation of sagittal adult spinal deformity after setting of fatigue post 10-minute walk. Neurospine 2021;18:495-503.




22. Kim JW, Kang MH, Oh JS. Patients with low back pain demonstrate increased activity of the posterior oblique sling muscle during prone hip extension. PM R 2014;6:400-5.



23. Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain 2000;85:317-32.


24. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31.



25. Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300-7.e2.


26. Meng NH, Li CI, Liu CS, et al. Comparison of height- and weight-adjusted sarcopenia in a Taiwanese metropolitan older population. Geriatr Gerontol Int 2015;15:45-53.


27. Kim YS, Lee Y, Chung YS, et al. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the Fourth Korean National Health and Nutritional Examination Surveys. J Gerontol A Biol Sci Med Sci 2012;67:1107-13.


28. Barbosa-Silva TG, Bielemann RM, Gonzalez MC, et al. Prevalence of sarcopenia among community-dwelling elderly of a medium-sized South American city: results of the COMO VAI? study. J Cachexia Sarcopenia Muscle 2016;7:136-43.


29. Moon BJ, Han MS, Kim JY, et al. Thoracolumbar slope is useful parameter for evaluating health-related quality of life and sagittal imbalance aggravation in adult spinal deformity: a prospective observational cohort study. Neurospine 2021;18:467-74.




30. Christie HJ, Kumar S, Warren SA. Postural aberrations in low back pain. Arch Phys Med Rehabil 1995;76:218-24.


31. Hansson T, Bigos S, Beecher P, et al. The lumbar lordosis in acute and chronic low-back pain. Spine (Phila Pa 1976) 1985;10:154-5.


32. Tsuji T, Matsuyama Y, Sato K, et al. Epidemiology of low back pain in the elderly: correlation with lumbar lordosis. J Orthop Sci 2001;6:307-11.


33. Murrie VL, Dixon AK, Hollingworth W, et al. Lumbar lordosis: study of patients with and without low back pain. Clin Anat 2003;16:144-7.


34. Pope MH, Bevins T, Wilder DG, et al. The relationship between anthropometric, postural, muscular, and mobility characteristics of males ages 18-55. Spine (Phila Pa 1976) 1985;10:644-8.


35. Konno S, Kikuchi S, Nagaosa Y. The relationship between intramuscular pressure of the paraspinal muscles and low back pain. Spine (Phila Pa 1976) 1994;19:2186-9.


36. Habibi Z, Maleki F, Meybodi AT, et al. Lumbosacral sagittal alignment in association to intervertebral disc diseases. Asian Spine J 2014;8:813-9.



37. Kjaer P, Leboeuf-Yde C, Korsholm L, et al. Magnetic resonance imaging and low back pain in adults: a diagnostic imaging study of 40-year-old men and women. Spine (Phila Pa 1976) 2005;30:1173-80.


38. Kim DH, Lee SY, Park SJ, et al. Relationships between spinal sarcopenia and spinal sagittal balance in older women. Ann Geriatr Med Res 2019;23:141.





- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2