|
 |
- Search
Abstract
A 32-year-old man presented with gradually increasing bilateral buttock pain. He had intermittent claudication. Multiple, homogenously enhanced intradural extramedullary lesions at L2-L3 and L5-S1 levels were observed on magnetic resonance imaging. The tumors were debulked and were removed in piecemeal pattern until they had completely been resected. Histopathological examination of the surgical specimens confirmed that both tumors were myxopapillary ependymomas (MPE). MPE presenting as concomitant double tumor at conus-cauda-filum level are very rare. This kind of presentation could not be directly considered as dissemination, since both tumors were in the site of classical origin of MPE. Ten cases of double spinal MPEs have been reported to date. Including the present case, analysis of the 11 patients revealed some facts. There is a male predominance, which is opposite to the ependymomas that are commonly observed in females. Median age at presentation is 15 years. Most pronounced symptom is low back pain that sometimes radiates to lower extremities. Surgical approach was aimed in all tumors, which could be succeeded in all tumors except one. Adjuvant radiation therapy was applied in 5 patients. No recurrences have been reported after surgery or surgery + radiotherapy regimens.
Glial cells are composed of astrocytes and ependymal cells. Astrocytes supply structural support of the central nervous system whereas ependymal cells line the ventricular system. Tumors arising from both cell lines compose most of the spinal intramedullary tumors. Myxopapillary ependymoma (MPE) is a subtype of ependymoma, which is exclusively observed at conus smedullaris, cauda equina, and filum terminale17).
Concomitant double tumor presentation of MPE at conuscauda-filum level is very rare. To date, 10 cases have been presented2,8,9,10,16,17,21). Herein, we report a new case of MPE presented as double tumor in noncontiguous segments of lumbosacral spinal column. We discuss clinical, radiological, pathological aspects and treatment modalities of these tumors.
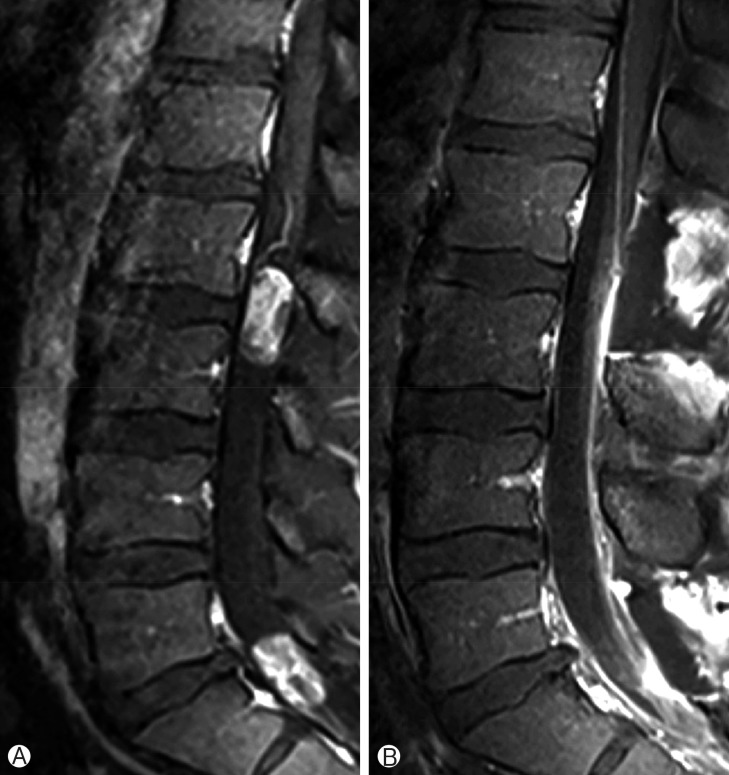
A 32-year-old man presented with gradually increasing bilateral buttock pain for the last 3 months. He had intermittent claudication. At examination, neither motor nor sensory disturbances were observed. Deep tendon reflexes were normal. No history of infection or trauma was present. The plain radiographs did not reveal any abnormalities. Magnetic resonance imaging showed multiple intradural extramedullary lesions with homogenous enhancement at L2-L3 and L5-S1 levels (Fig. 1). Whole cranium and spine were searched for any other tumors, yet no other one could be observed except the ones we have already detected at the lumbosacral region. L2 and L5 laminectomy were performed at the same session. Both lesions were observed as rounded, soft and reddish-gray colored. The lesions were completely encapsulated by thin fibrous capsules with a well-delimitated cleavage plane. The tumors were removed in piecemeal pattern until they were completely resected under the surgical microscope. Following resection and hemostasis, the dura was repaired in watertight fashion. Postoperative imaging confirmed total resection of both lesions (Fig. 2). Histopathological examination of the surgical specimens confirmed that both lesions were MPEs (Fig. 3). No adjuvant therapies were performed. At 1-year follow-up his neurological status remained stable.
MPEs are World Health Organization grade I (low-grade) ependymomas originating mostly from the filum terminale or the terminal ventricle5,14,19). For this reason MPEs are detected as solitary tumors more commonly at conus-cauda-filum levels5,19,21). Intracranial, cervical and thoracic region lesions are considered as CSF dissemination of MPEs, yet reverse situations have also been reported21,25). Tumor dissemination is basically caused by violation of tumor capsule of MPEs caused by piecemeal tumor removal, traumatic spinal tap, spinal trauma, spinal instrumentation, and tumor hemorrhage4,11,15,18,20,21,23,27).
MPE is iso-intense on T1- and hyper-intense on T2-weighted MRI with heterogenous imaging properties on T2-weighted scans. These heterogeneities correspond to hemorrhages and calcifications present in the tumor3,12).
MPE is composed of cellular areas harboring rosettes and pseudorosettes intermingled with papillary regions, which have vascular cores in mucoid matrix14,19,21). MPEs sometimes coexist with spinal dysraphisms1,7,24,26).
MPEs presenting as concomitant double tumors at conuscauda-filum level are very rare. This kind of presentation could not be directly considered as dissemination, since both tumors are in the site of classical origin of MPEs21). Ten cases have been presented up to date (Table 1)2,8,9,10,16,17,21). Including the present case, analyses of the 11 patients have revealed some facts. There is a male predominance (male:female=6:1; sex was not mentioned for 4 patients10)), which is opposite to the ependymomas which are commonly observed in females6). Median age at presentation is 15 years (range, 7-39 years; age was not mentioned for 4 patients10)). This depicts that concomitant double presentation of MPE is mostly observed in children and young adults. Most common symptom is low back pain, sometimes radiating to lower extremity(ies). Surgical approach was aimed in all tumors, which was successful in all tumors, except one. Adjuvant radiation therapy was performed in 5 patients. In the first patient, both tumors had been resected en bloc, yet MIB-1 index was high (9.1%). Whole craniospinal radiotherapy was given to the patient2). In the second patient, the second tumor could be excised partially, and local radiotherapy was given to the residual tumor bed16). Khalatbari et al.10) operated 4 patients (6 tumors were removed en bloc, 3 tumors were removed gross totally in piecemeal fashion) and gave adjuvant radiotherapy to 3 patients. Average follow-up time was 3.4 years without any recurrence in any case.
MPEs have 3.6 times shorter time to metastasis compared to other type of low-grade ependymomas. Dissemination is more commonly seen in young patients with ependymomas20). So, it is not clear yet if concomitant double tumor presentation is due to two different primary tumors or dissemination one from the other one17). The second lesions have mainly been observed at the lumbosacral junction, which might be due to drop metastasis from the rostral lesion2,8,9,16,17,21).
MPEs histologically have low malignancy level. However, tumor dissemination is observed in 33% of patients with MPE at 5-year follow-up20). So, long-term follow-up should be conveyed in every patient with a diagnosis of MPE, even in cases with aggressive therapy13,23). Good prognosis is expected in 70% of the patients with appropriate treatment without considering total resection as a must2,22,23).
CONFLICT OF INTEREST
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
1. Adamson DC, Cummings TJ, Friedman AH. Myxopapillary ependymoma and fatty filum in an adult with tethered cord syndrome: a shared embryological lesion? Case report. Neurosurgery 2005 57:E373PMID: 16094143.


2. Andoh H, Kawaguchi Y, Seki S, Asanuma Y, Fukuoka J, Ishizawa S, et al. Multi-focal myxopapillary ependymoma in the lumbar and sacral regions requiring cranio-spinal radiation therapy: a case report. Asian Spine J 2011 5:68-72. PMID: 21386949.



3. Bandopadhayay P, Silvera VM, Ciarlini PD, Malkin H, Bi WL, Bergthold G, et al. Myxopapillary ependymomas in children: imaging, treatment and outcomes. J Neurooncol 2015 [Epub ahead of print].

4. Chan HS, Becker LE, Hoffman HJ, Humphreys RP, Hendrick EB, Fitz CR, et al. Myxopapillary ependymoma of the filum terminale and cauda equina in childhood: report of seven cases and review of the literature. Neurosurgery 1984 14:204-210. PMID: 6709144.


5. Choi BH, Kim RC, Suzuki M, Choe W. The ventriculus terminalis and filum terminale of the human spinal cord. Hum Pathol 1992 23:916-920. PMID: 1644436.


6. Duffau H, Gazzaz M, Kujas M, Fohanno D. Primary intradural extramedullary ependymoma: case report and review of the literature. Spine (Phila Pa 1976) 2000 25:1993-1995. PMID: 10908946.


7. Gallia GL, Burger PC, Suk I, Bagley CA, Wolinsky JP, Garonzik IM, et al. Concomitant conus medullaris ependymoma and filum terminale lipoma: case report. Neurosurgery 2006 58:E1214PMID: 16723873.


8. Gelabert-González M, Prieto-González A, Abdulkader-Nallib I, Cutrín-Prieto J. Double ependymoma of the filum terminale. Childs Nerv Syst 2001 17:106-108. PMID: 11219615.


9. Hallacq P, Labrousse F, Streichenberger N, Lisii D, Fischer G. Bifocal myxopapillary ependymoma of the terminal filum: the end of a spectrum? Case report. J Neurosurg 2003 98(3 Suppl):288-289. PMID: 12691387.


10. Khalatbari MR, Hamidi M, Moharamzad Y. Primary multi-focal myxopapillary ependymoma of the filum terminale. J Neurosurg Sci 2015 [Epub ahead of print].

11. Khalatbari MR, Jalaeikhoo H, Hamidi M, Moharamzad Y. Craniospinal dissemination of filum myxopapillary ependymoma following spinal trauma: case report and literature review. Childs Nerv Syst 2013 29:149-152. PMID: 23011448.


12. Klekamp J. Spinal ependymomas. Part 2: Ependymomas of the filum terminale. Neurosurg Focus 2015 39:E7PMID: 26235024.

13. Landriel F, Ajler P, Tedesco N, Bendersky D, Vecchi E. Multicentric extramedullary myxopapillary ependymomas: Two case reports and literature review. Surg Neurol Int 2012 3:102PMID: 23087818.



14. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007 114:97-109. PMID: 17618441.



15. Martinez-Perez R, Hernandez-Lain A, Paredes I, Munarriz PM, Castaño-Leon AM, Lagares A. Acute neurological deterioration as a result of two synchronous hemorrhagic spinal ependymomas. Surg Neurol Int 2012 3:33PMID: 22530168.



16. McLaughlin N, Guiot MC, Jacques L. Double myxopapillary ependymomas of the filum terminale. Can J Neurol Sci 2011 38:151-154. PMID: 21156449.


17. Nakama S, Higashi T, Kimura A, Yamamuro K, Kikkawa I, Hoshino Y. Double myxopapillary ependymoma of the cauda equina. J Orthop Sci 2005 10:543-545. PMID: 16193371.


18. Plans G, Brell M, Cabiol J, Villá S, Torres A, Acebes JJ. Intracranial retrograde dissemination in filum terminale myxopapillary ependymomas. Acta Neurochir (Wien) 2006 148:343-346. PMID: 16362177.


19. Rasmussen TB, Kernohan JW, Adson AW. pathologic classification, with surgical consideration, of intraspinal tumors. Ann Surg 1940 111:513-530. PMID: 17857556.



20. Rezai AR, Woo HH, Lee M, Cohen H, Zagzag D, Epstein FJ. Disseminated ependymomas of the central nervous system. J Neurosurg 1996 85:618-624. PMID: 8814165.


21. Salomão JF, de Andrade CV, Bellas AR, Cervante TP. The nature of double concomitant myxopapillary ependymoma: report of a case. Childs Nerv Syst 2014 30:527-530. PMID: 23904042.


22. Schweitzer JS, Batzdorf U. Ependymoma of the cauda equina region: diagnosis, treatment, and outcome in 15 patients. Neurosurgery 1992 30:202-207. PMID: 1545888.


23. Sonneland PR, Scheithauer BW, Onofrio BM. Myxopapillary ependymoma: a clinicopathologic and immunocytochemical study of 77 cases. Cancer 1985 56:883-893. PMID: 4016681.


24. Tubbs RS, Kelly DR, Mroczek-Musulman EC, Braune K, Reddy A, Georgeson K, et al. Dwarfism, occult spinal dysraphism, and presacral myxopapillary ependymoma with an epidermoid cyst in a child. Acta Neurochir (Wien) 2005 147:299-302. PMID: 15662566.


25. Wang M, Wang H, Zhou Y, Zhan R, Wan S. Myxopapillary ependymoma in the third ventricle area and sacral canal: dropped or retrograde metastasis? Neurol Med Chir (Tokyo) 2013 53:237-241. PMID: 23615415.


26. Wolf NI, Harting I, Hartmann M, Aschoff A, Sommer C, Rating D, et al. Combination of caudal myxopapillary ependymoma and dermal sinus: a single shared embryologic lesion? Dev Med Child Neurol 2003 45:568-570. PMID: 12882537.


27. Yücesoy K, Ozer E, Koyuncuoglu M. Parenchymal brain metastasis of a spinal myxopapillary ependymoma after extradural manipulation. Acta Neurochir (Wien) 2001 143:1071-1072. PMID: 11685616.


Fig. 1
One lesion at L2-L3 level and other lesion at L5-S1 level is observed through the tight filum terminale on T2-weighed magnetic resonance imaging scan (A). (B) Homogenous gadolinium enhancement is observed in both pathologies.
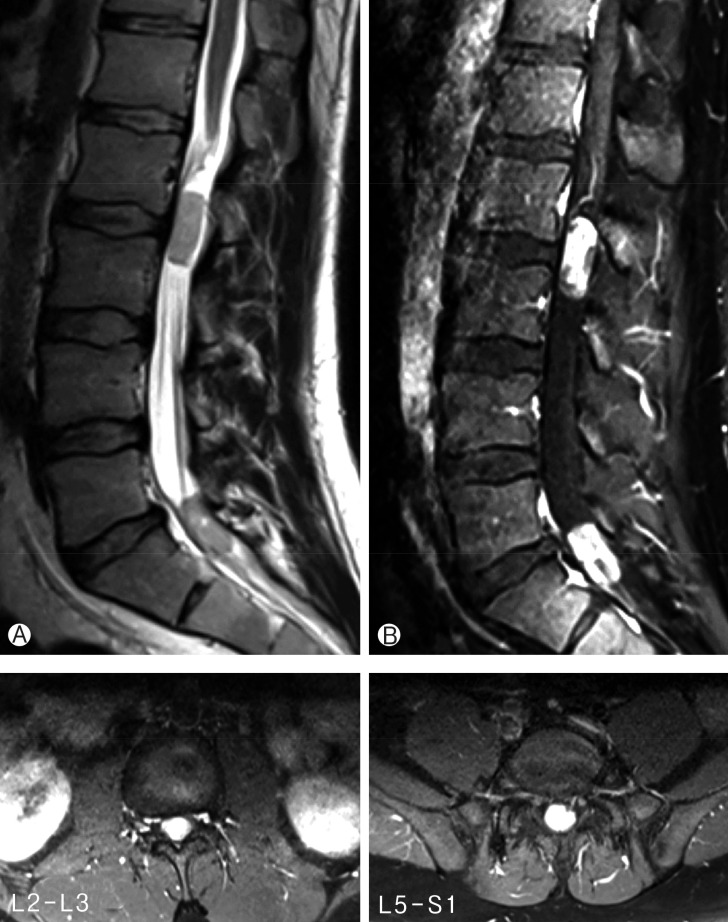
Fig. 2
Both lesions were resected totally: preoperative (A), and postoperative (B) contrast enhanced T1-weighted lumbar magnetic resonance imaging scans.